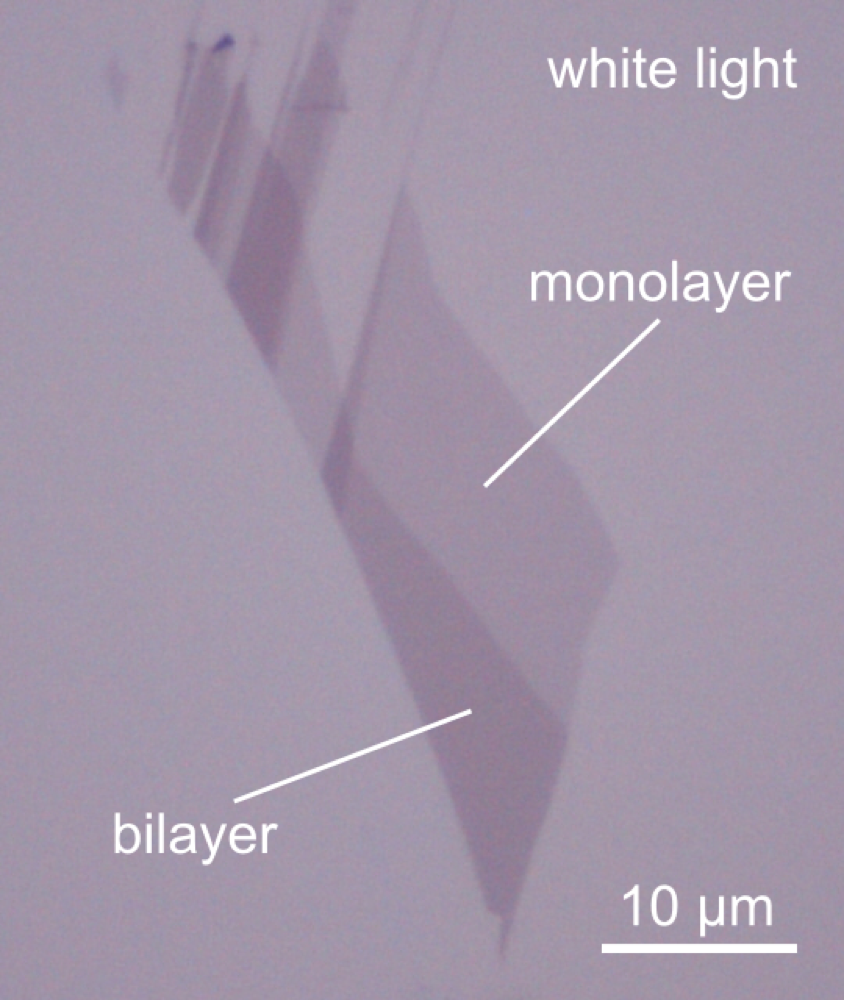
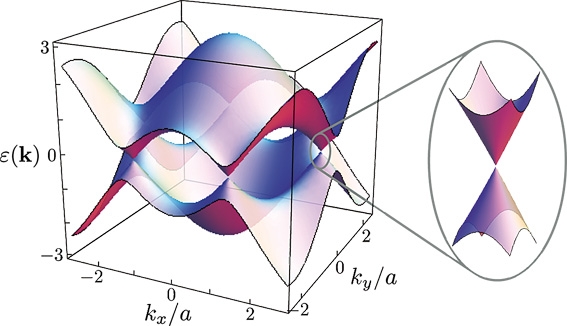
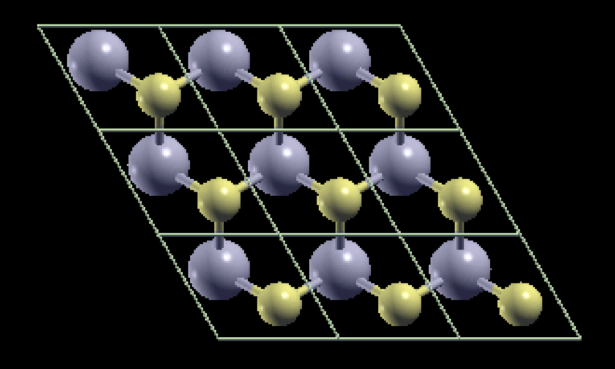
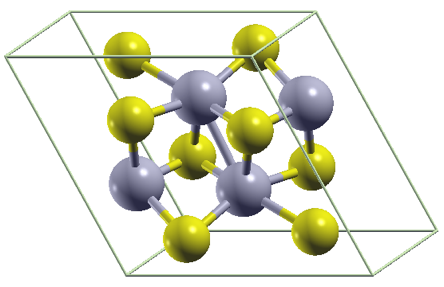
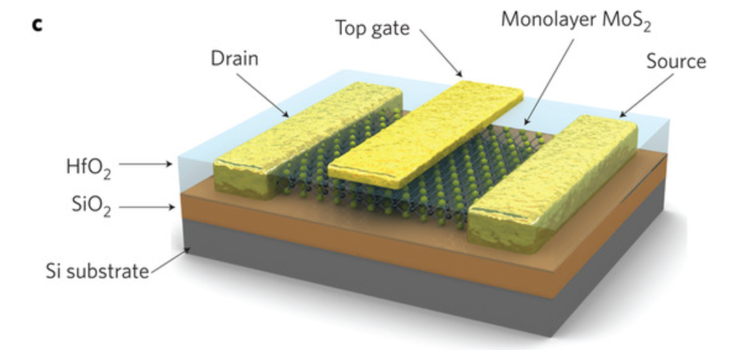
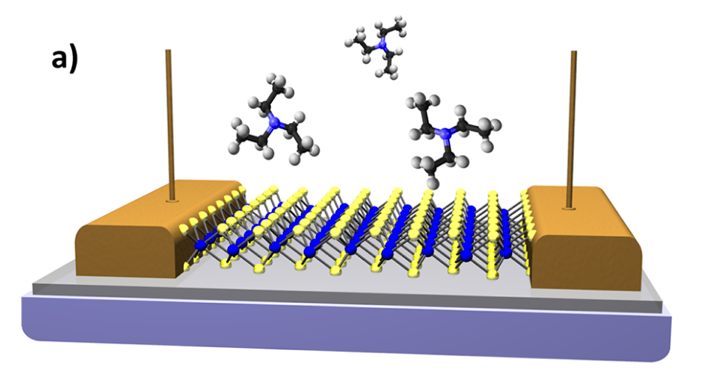
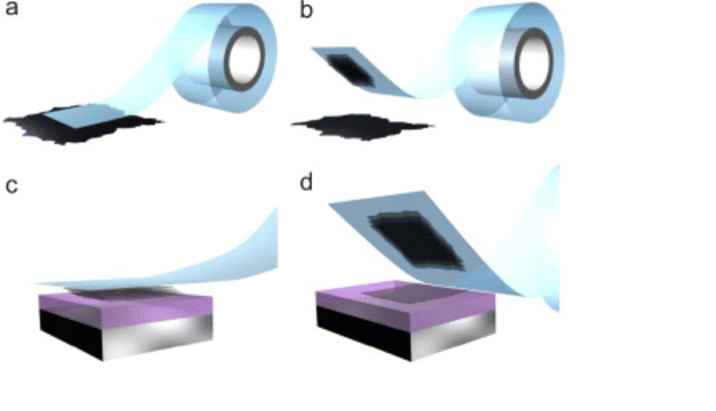
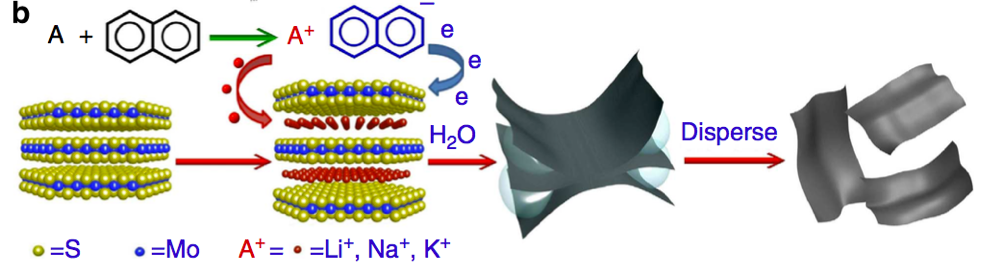
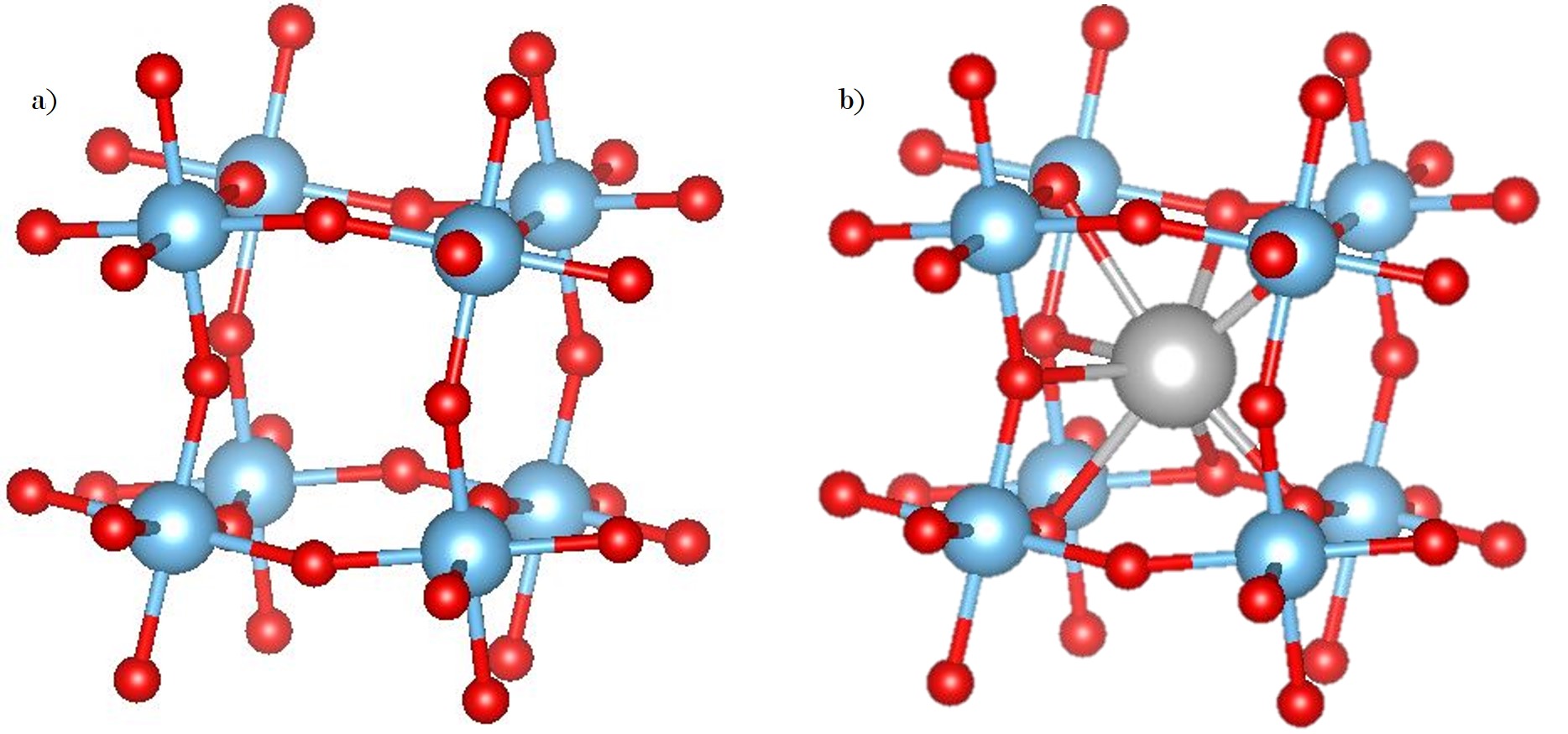
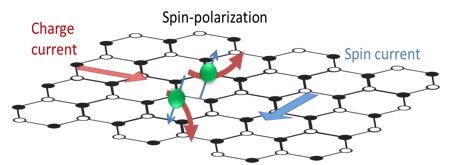
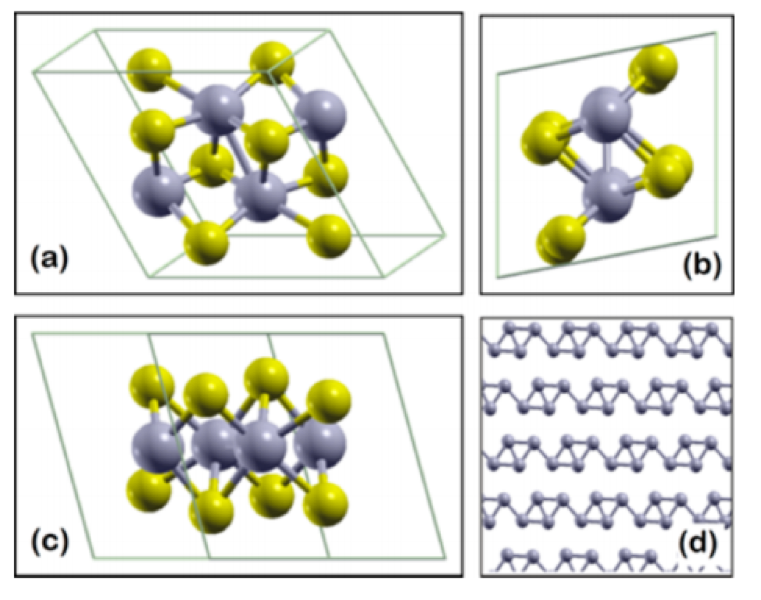
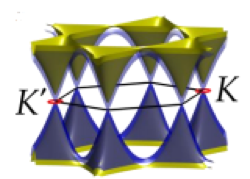
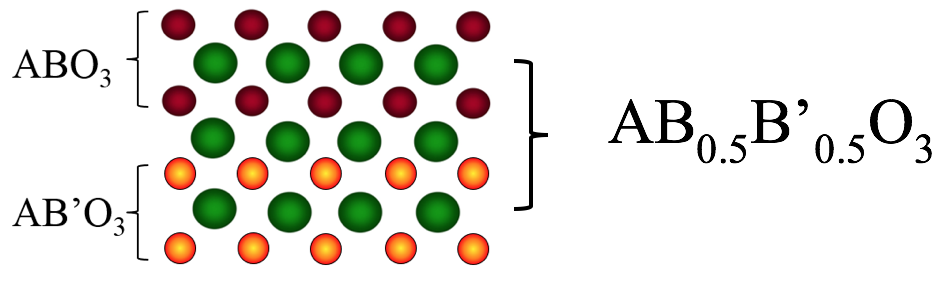WHAT IS 2D?
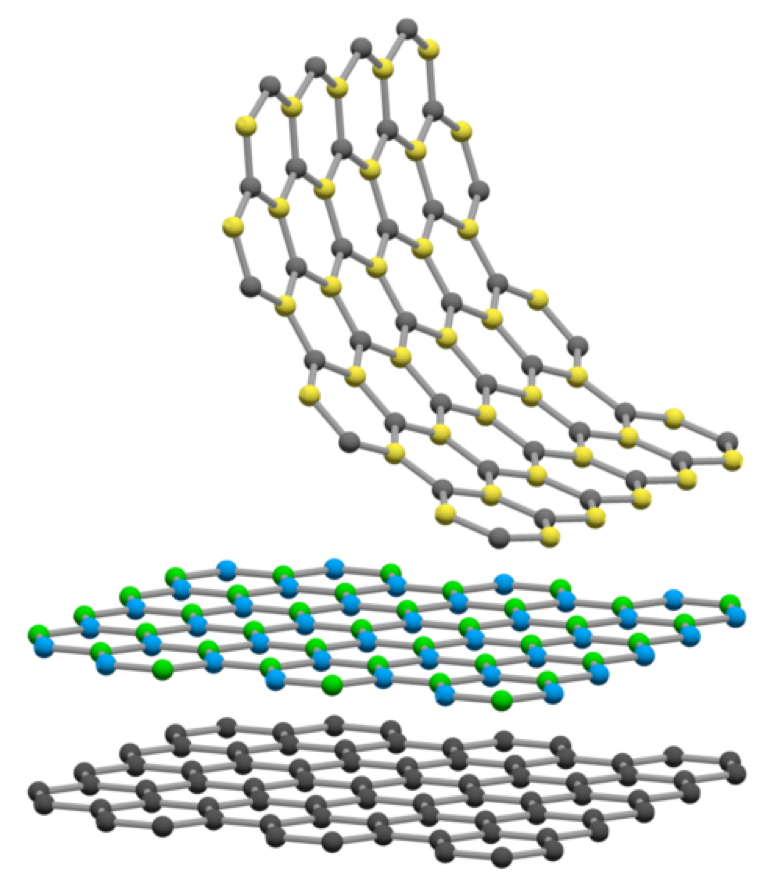
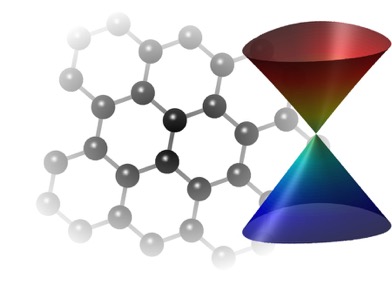
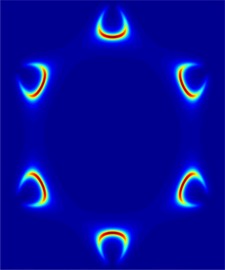
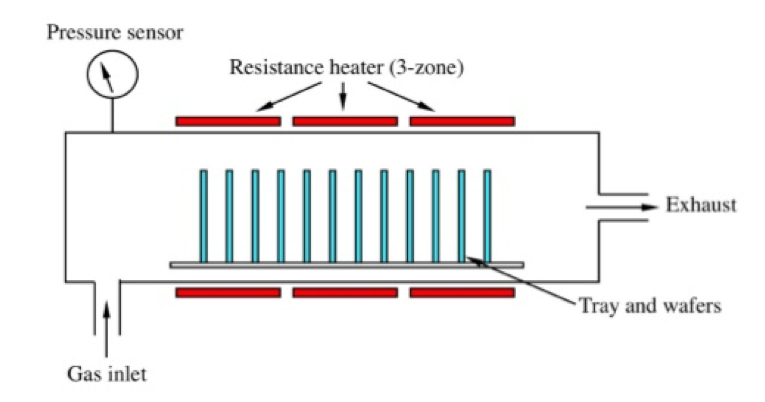
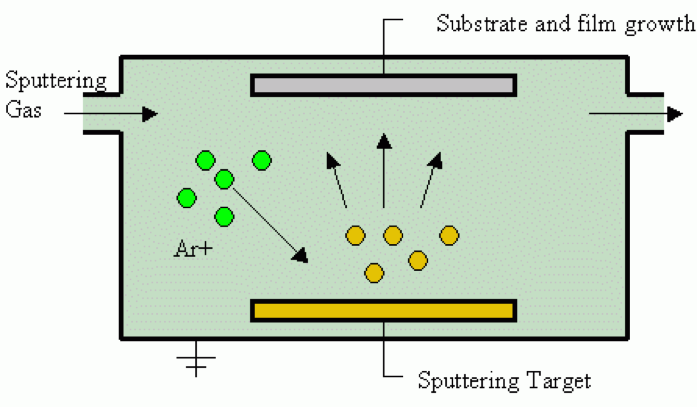
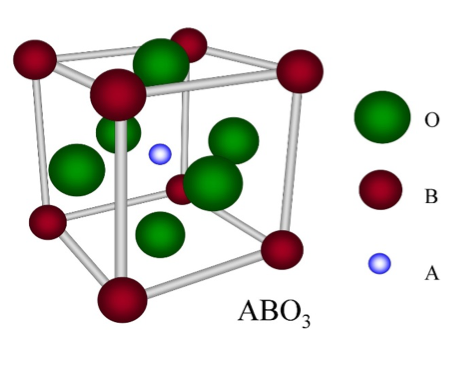
Determining which materials can be considered as two-dimensional is not clearly defined. A strict definition could limit this class of materials to the crystalline materials consisting of only a single layer of atoms. This would limit the discussion to graphene-like materials and transition metal dichalcogenides (TMDs). In photonics however, 2D materials are all materials whose thicknesses are considerably smaller than the wavelength of the light used. Another definition could be any material where the physics is constricted in one of the spatial dimensions, since the properties of thin films can differ from their bulk equivalents. For superconducting materials, the thickness becomes relevant if it is smaller than the materials’ penetration depth. In order to keep the subject broad, we have decided to include any materials where the reduced dimensionality influences the material’s properties. This includes materials with interesting surface or interface physics, few-layered materials and thin films.